1. General attack corrosion
This very common form of corrosion, attacks the entire surface of a metal structure. It is caused by chemical or electrochemical reactions. While general attack corrosion can cause a metal to deteriorate to point of failure, it is also a known and predictable issue. As a result, it is possible to plan for and manage general attack corrosion.

2. Localised corrosion
Localised corrosion attacks only specific areas of a metal structure. There are three types of localised corrosion:
Pitting corrosion
Is a form of corrosion which results in holes or cavities within a metal.
Crevice corrosion
Corrosion that occurs in stagnant water often at joints, such as bolted connections.
Filiform corrosion
Occurs beneath coatings where humidity of circa 75%, and moisture and oxygen permeate beneath the coating.
3. Galvanic corrosion:
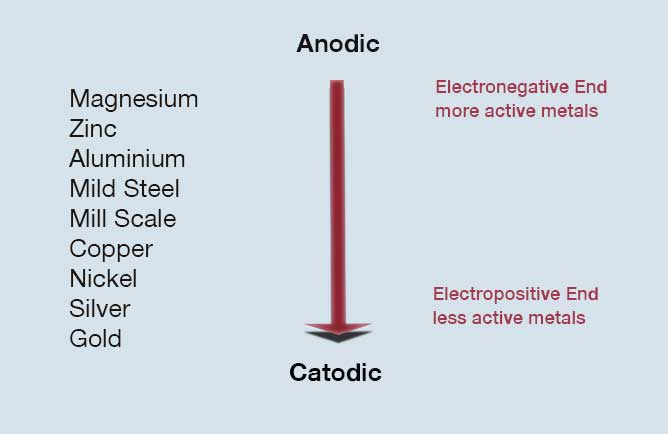 Galvanic corrosion normally occurs where two dissimilar metals are in direct electrical contact in the presence of an electrolyte which might commonly be condensation, rain water or sea water. Effectively a battery cell is created and an electrical current flows as a result of the corrosion of one of the two metals.
Galvanic corrosion normally occurs where two dissimilar metals are in direct electrical contact in the presence of an electrolyte which might commonly be condensation, rain water or sea water. Effectively a battery cell is created and an electrical current flows as a result of the corrosion of one of the two metals.
In essence, one metal’s molecules are drawn toward the other, leading to corrosion in only one of the two metals. It is the more electronegative metal in the electrochemical series (image 2) which corrodes. Read more about galvanic corrosion.



 Galvanic corrosion normally occurs where two dissimilar metals are in direct electrical contact in the presence of an electrolyte which might commonly be condensation, rain water or sea water. Effectively a battery cell is created and an electrical current flows as a result of the corrosion of one of the two metals.
Galvanic corrosion normally occurs where two dissimilar metals are in direct electrical contact in the presence of an electrolyte which might commonly be condensation, rain water or sea water. Effectively a battery cell is created and an electrical current flows as a result of the corrosion of one of the two metals.