 During the hot dip galvanizing process, iron and steel components are heated to the temperature of the galvanizing bath, of about 450°C. While the steel is immersed in the molten zinc, layers of zinc-iron alloy are formed on the surface by a process of diffusion.
During the hot dip galvanizing process, iron and steel components are heated to the temperature of the galvanizing bath, of about 450°C. While the steel is immersed in the molten zinc, layers of zinc-iron alloy are formed on the surface by a process of diffusion.
Galvanized Steel Composition
When the steel components are withdrawn from the bath these alloy layers are often covered with a coating of pure zinc.
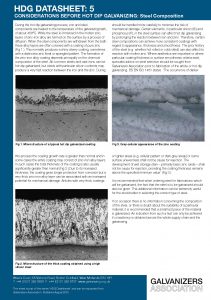
The formation of the zinc-iron alloy coating depends principally on the chemical composition of the steel that is galvanized. All common steels and irons can be hot dip galvanized, but steels with particular silicon contents may produce a very fast reaction between the iron and the zinc.
Want to have a pdf version of this datasheet? Click the button below.
Certain elements, in particular silicon (Si) and phosphorus (P), in the steel surface can affect hot dip galvanizing by prolonging the reaction between iron and zinc.
Therefore, certain steel compositions can achieve more consistent coatings with regard to appearance, thickness and smoothness. The prior history of the steel can also affect its reaction with molten zinc.
It is recommended that when ordering steel for fabrications which will be galvanized, the fact that the steel is to be galvanized should also be given. This additional information can be extremely useful for the stockholder in selecting the correct type of steel.
Download the datasheet for more information.

